Breakthrough Heart Disease Treatments by Cardiol Therapeutics
Investor Insight
Cardiol Therapeutics is positioned with a late-stage lead asset, multiple orphan indications, strong clinical proof-of-concept data, and a differentiated non-immunosuppressive approach to inflammatory heart disease. The company’s 2025–2026 catalysts – MAVERIC Phase III enrollment progress, full ARCHER data, and CRD-38 clinical initiation – are key value drivers in large and underserved cardiovascular markets.
Overview
Cardiol Therapeutics Inc. (NASDAQ:CRDL,TSX:CRDL) is a clinical-stage life sciences company focused on the research, development, and commercialization of innovative anti-inflammatory and anti-fibrotic therapies for the treatment of heart disease. The company’s programs target serious, often life-threatening cardiovascular conditions for which there are limited or no approved treatments, including recurrent pericarditis, acute myocarditis, and heart failure.
Cardiol’s therapeutic approach centers on modulating inflammasome pathway activation—a central driver of inflammation and fibrosis in the heart. This is achieved through pharmaceutically manufactured cannabidiol formulations developed under cGMP standards. Cannabidiol has been shown in preclinical and clinical studies to down-regulate inflammatory mediators (e.g., IL-1, IL-6) and preserve cardiac structure and function, offering the potential for disease-modifying, non-immunosuppressive treatment options.
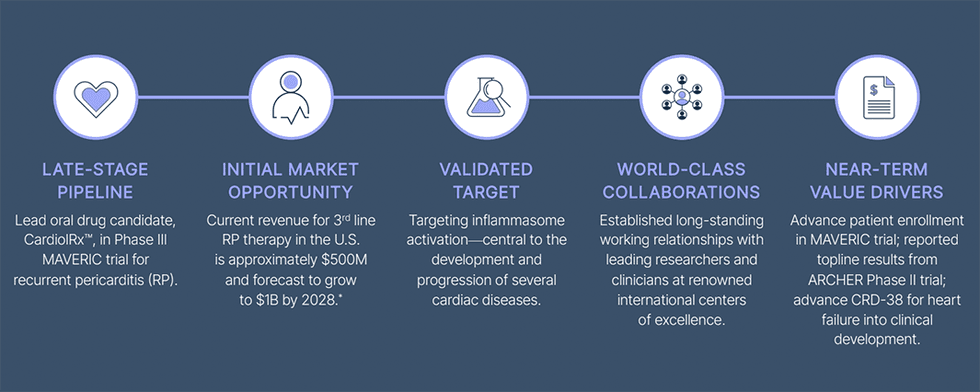
The company’s lead oral drug candidate, CardiolRx™, is in late-stage clinical development:
Cardiol is also advancing CRD-38, a proprietary subcutaneous cannabidiol formulation for heart failure. IND-enabling studies are underway following compelling preclinical results showing cardioprotection through preservation of mitochondrial function and prevention of cardiac remodeling.

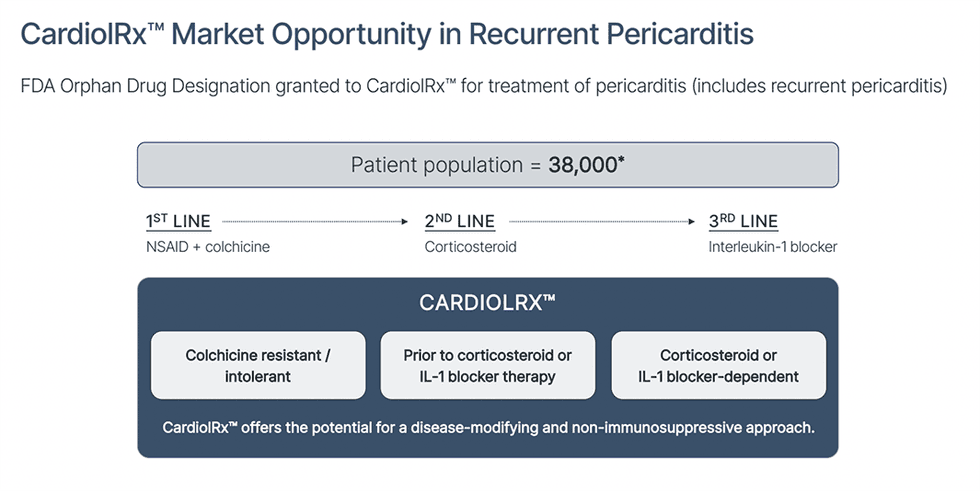
What it is: A pivotal clinical program testing CardiolRx™ in patients with recurrent pericarditis, a painful and debilitating inflammation of the membrane around the heart that often returns despite prior treatments. The condition can cause severe chest pain, shortness of breath, and repeated hospitalizations.
Why it matters: Current third-line therapy is costly, immunosuppressive and associated with a high recurrence rate after discontinuation. CardiolRx™ offers the potential for a non-immunosuppressive, disease-modifying option.
Status:
- Completed MAvERIC-Pilot Phase II: Rapid and durable reductions in pain and inflammation sustained over 26 weeks; majority of patients recurrence-free during extension.
- Phase III MAVERIC trial: Multinational, double-blind, placebo-controlled study enrolling 110 high-risk patients across ~20 sites in North America and Europe. Primary endpoint: recurrence-free rate at 24 weeks after IL-1 blocker discontinuation.
What it is: A global Phase II trial evaluating CardiolRx™ in acute myocarditis, an inflammatory heart…
Read More: Breakthrough Heart Disease Treatments by Cardiol Therapeutics
